We have shared detailed notes of Class 9th Science NCERT chapter Structure Of Atoms to help the students with the lesson and grab the conceptual knowledge of Chemistry. We have also shared NCERT Solutions for class 9th Structure Of Atoms.
We have also shared some important diagrams to support the concepts and give a nice briefing of the lesson Structure of Atoms Class 9th Science NCERT.
Structure of Atoms Class 9th Science: Introduction
Atoms are not indivisible and are composed of three fundamental particles. These particles are electrons, protons, and neutrons.
Charged particles in Matter
o Electrons are negatively-charged particles. They were discovered by J. J. Thomson, in
a cathode ray experiment.
o Canal rays are positively charged radiation consisting of protons. Protons are positive-
charged particles and were discovered by E. Goldstein.
o The third fundamental particles present in an atom are neutrons. They are electrical-
neutral and were discovered by J. Chadwick.
Various models were given to explain the structure of atoms-
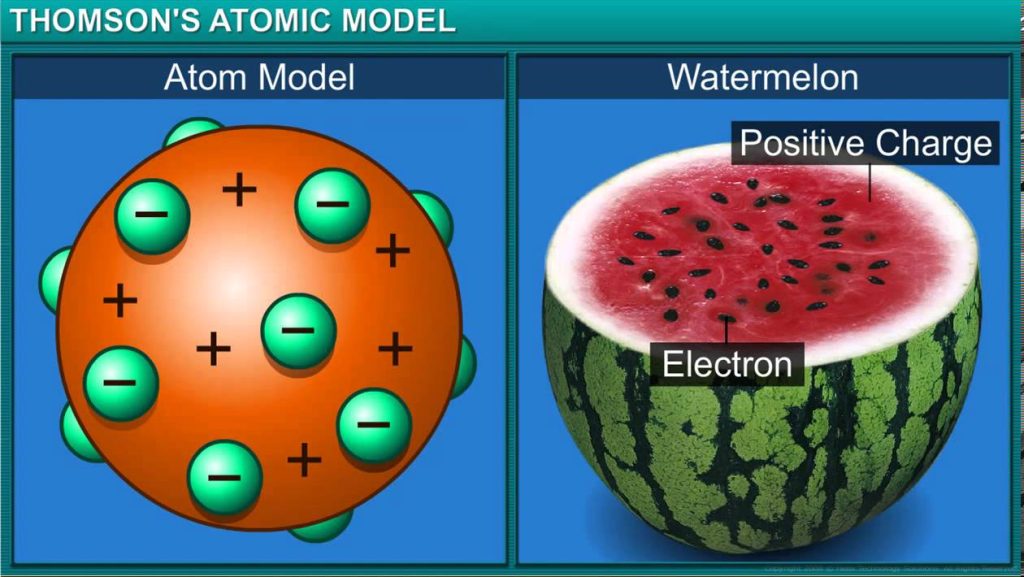
Thomson’s atomic model:
o Thomson thought that an atom is a sphere of positive charge in which electrons are
embedded.
o An atom as a whole is electrically neutral because the negative and positive charges are
equal in magnitude.
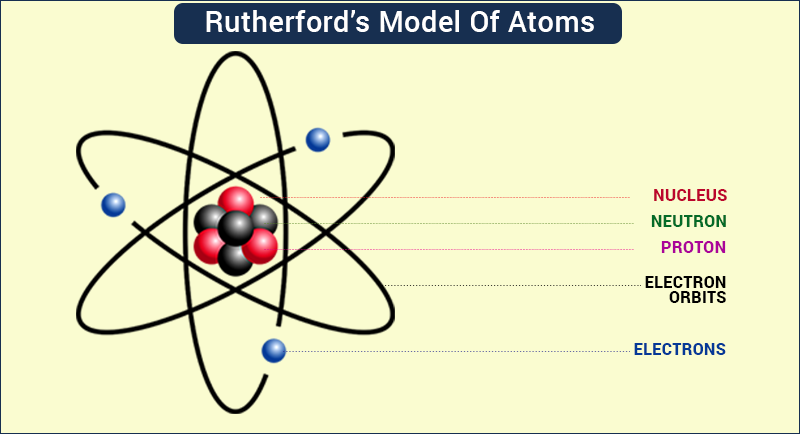
Rutherford’s atomic model:
o On the basis of his experiments with alpha rays and gold foil, Rutherford concluded that
Thomson’s atomic model was incorrect.
o He proposed an atomic model based on the results of his experiments.
o In this model, all the positive charges (i.e., protons) were present at the center of the atom, inside the nucleus, and the electrons were present in circular orbits around the nucleus.
o He said that the electrons are not at rest and keep moving continuously in these circular
orbits.
o He also said that the size of the nucleus is very small as compared to that of the atom.
Drawbacks of Rutherford’s Model
o It cannot explain the stability of an atom on the basis of classical mechanics and
electromagnetic theory.
o If the electrons were stationary, then the strong electrostatic force of attraction between
the dense nucleus and the electrons would pull the electrons towards the nucleus. Thus, it
cannot explain the stability of an atom.
o Rutherford’s model does not give any idea about the distribution of electrons around the nucleus (i.e., the electronic structure of the atom), and about their energy.
o It cannot explain the atomic spectra.
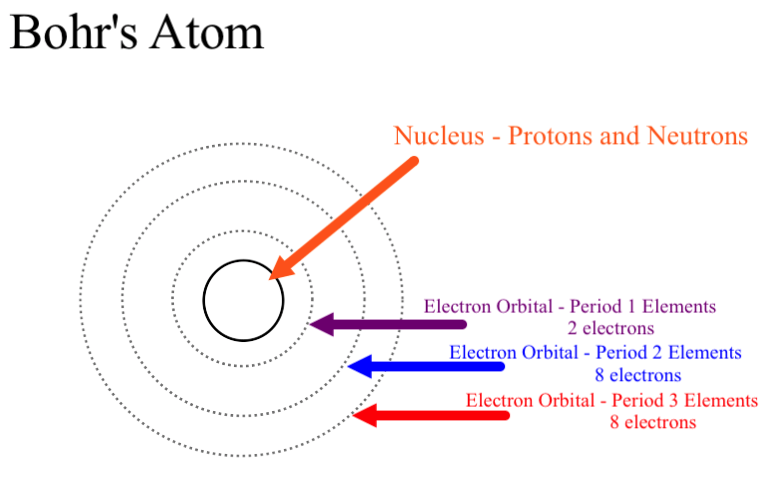
Bohr’s atomic model:
o Neils Bohr proposed that the electrons present around the nucleus revolve in specific
orbits called energy levels.
o He also stated that the electrons do not release energy while revolving. Thus, the resulting atom is a stable one.
o The shells in which the electrons are present are known as K, L, M, N, and so on (or 1, 2, 3, 4, and so on), as proposed by Bohr and Bury.
o Each shell contains a specific number of electrons, which can be calculated using the formula 2n2.
Atomic Models
- Dalton’s atomic model Thomson’s atomic model
- Rutherford’s atomic model Bohr’s atomic model
o Valency is defined as the combining capacity of the atom of an element. The valence of an element depends upon the number of electrons present in the outermost shell of its atom.
An atomic number of an element is equal to the number of protons present in the atom and atomic mass is equal to the sum of the number of protons and neutrons present in it.
Isotopes are atoms having the same atomic number and different atomic masses.
Isobars are atoms having the same atomic mass and different atomic numbers.
NCERT Solutions For Class 9th Structure Of Atoms
We have provided ncert solutions for class 9th structure of atoms to help the students with the ncert solutions so that students are able to grasp the concept of the structure of atoms and be accurate with the solutions thereafter.
Q1. What are canal rays?
Canal rays are positively charged radiations. These rays consist of positively charged particles known as protons. They were discovered by Gold stein in 1886.
Q2. If an atom contains one electron and one proton, will it carry any charge or not?
An electron is a negatively charged particle, whereas a proton is a positively charged particle. The magnitude of their charges is equal. Therefore, an atom containing one electron and one proton will not carry any charge. Thus, it will be a neutral atom.
Q3. On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
According to Thomson’s model of the atom, an atom consists of both negatively and positively charged particles. The negatively charged particles are embedded in the positively charged sphere. These negative and positive charges are equal in magnitude. Thus, by counterbalancing each other’s effect, they make an atom neutral.
Q4. On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
On the basis of Rutherford’s model of an atom, protons (positively-charged particles) are present in the nucleus of an atom.
Q5. What do you think would be the observation if the α-particle scattering experiment is carried out using a foil of a metal other than gold?
If the α-scattering experiment is carried out using a foil of metal rather than gold, there would be no change in the observation. In the α-scattering experiment, a gold foil was taken because gold is malleable and a thin foil of gold can be easily made. It is difficult to make such foils from other metals.
Q6. Name the three sub-atomic particles of an atom.
The three sub-atomic particles of an atom are:
(i) Protons
(ii) Electrons, and
(iii) Neutrons
Q7. The helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Helium atom has two neutrons. The mass of an atom is the sum of the masses of protons and neutrons present in its nucleus.
Since the helium atom has two protons, the mass contributed by the two protons is (2 × 1) u = 2 u. Then, the remaining mass (4 − 2) u = 2 u is contributed by 2u /1u = 2 neutrons.
Q8. How will you find the valency of chlorine, sulfur, and magnesium?
If the number of electrons in the outermost shell of the atom of an element is less than or equal to 4, then the valency of the element is equal to the number of electrons in the outermost shell.
On the other hand, if the number of electrons in the outermost shell of the atom of an element is greater than 4, then the valency of that element is determined by subtracting the number of electrons in the outermost shell from 8.
The distribution of electrons in chlorine, sulfur, and magnesium atoms are 2, 8, 7; 2, 8, 6, and 2, 8, 2 respectively. Therefore, the number of electrons in the outermost shell of chlorine, sulfur, and magnesium atoms are 7, 6, and 2 respectively.
Thus,
The valency of chlorine = 8 −7 = 1
The valency of sulphur = 8 − 6 = 2
The valency of magnesium = 2
Q9. If the number of electrons in an atom is 8 and the number of protons is also 8, then
(i) what is the atomic number of the atom and
(ii) what is the charge on the atom?
(i) The atomic number is equal to the number of protons. Therefore, the atomic number of
the atom is 8.
(ii) Since the number of both electrons and protons is equal, therefore, the charge on the
atom is 0.
Q9. Compare the properties of electrons, protons, and neutrons.
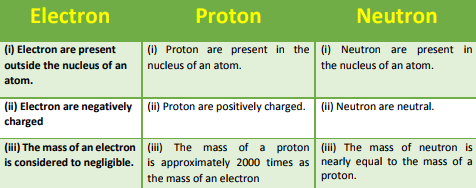
Q10. What are the limitations of Rutherford’s model of the atom?
According to Rutherford’s model of an atom, electrons revolve around the nucleus in fixed orbits. But, an electron revolving in circular orbits will not be stable because, during the revolution, it will experience acceleration. Due to acceleration, the electrons will lose energy in the form of radiation and fall into the nucleus. In such a case, the atom would be highly unstable and collapse.
Q11. Define valency by taking examples of silicon and oxygen.
The valency of an element is the combined capacity of that element. The valency of an element is determined by the number of valence electrons present in the atom of that element.
If the number of valence electrons of the atom of an element is less than or equal to four, then the valency of that
element is equal to the number of valence electrons. For example, the atom of silicon has four valence electrons.
Thus, the valency of silicon is four. On the other hand, if the number of valence electrons of the atom of an element is greater than four, then the valency of that element is obtained by subtracting the number of valence electrons from eight. For example, the atom of oxygen has six valence electrons. Thus, the valency of oxygen is (8 − 6) i.e., two.
Q12. If a bromine atom is available in the form of, say, two isotopes Br 35 79 (49.7%) and Br 35 81 (50.3%), calculate the average atomic mass of the bromine atom.
The average atomic mass of bromine
= (79 x 49.7)+ (81 x 50.3)/100
= (3926.3 + 4074.3)/100
= 8000.6/100
= 80 u
Q13. The average atomic mass of a sample of element X is 16.2 u. What are the percentages of isotopes 8X 16 and 8X 18 in the sample?
Since average atomic mass
= 16 x X + 18 x (100 – X )/100
16.2 = 16X + 1800 – 18X/100
1620 = – 2X + 1800
2X = 1800 – 1620
X = 180/2 = 90
When 90% is the X-16 sample so for X-18 sample % = 100-90=10%
Q14. For the following statements, write T for ‘True’ and F for ‘False’.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of a proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons. (F)
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral. (F)
(c) The mass of an electron is about 1/2000 times that of a proton. (T)
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine. (T)
Conclusion
We have shared all about class 9th science NCERT chapter Structure Of Atoms along with NCERT Solutions for class 9th Structure Of Atoms to help the students of class 9th with the concepts of the structure of atoms and ncert solutions to give the pristine knowledge of the chapter.
Related Article